As the world transitions toward renewable energy, water electrolysis has become a cornerstone of green hydrogen production. The choice of electrode material significantly impacts both the efficiency and sustainability of this process. Among the most commonly used materials are Platinum (Pt), Iridium Oxide (IrO₂), Ruthenium Oxide (RuO₂), Nickel (Ni), and Mixed Metal Oxides (MMOs). This article explores these materials, considering their performance, economic viability, and environmental impact.
Detailed Analysis of Anode Materials for Water Electrolysis
The selection of anode materials for water electrolysis is critical for achieving high efficiency, durability, and stability. Below is a comprehensive analysis of the properties of common anode materials, focusing on their color, chemical characteristics, physical attributes, water electrolysis efficiency, and bonding efficiency with titanium substrates.
1. Platinum (Pt)
- Color:
Platinum exhibits a distinct silver-white metallic color, which is visually appealing and indicative of its purity. - Chemical Characteristics:
Platinum is one of the most chemically stable elements. It is highly resistant to oxidation and corrosion, making it suitable for both acidic and alkaline environments. - Physical Characteristics:
Platinum has excellent electrical and thermal conductivity, high density (21.45 g/cm³), and a melting point of 1768°C. These properties contribute to its superior performance in harsh electrolytic conditions. - Water Electrolysis Efficiency:
Platinum offers exceptional catalytic activity for the oxygen evolution reaction (OER), with one of the lowest overpotentials among all materials. This makes it highly efficient but comes with a significant cost constraint due to its rarity. - Bonding with Titanium Substrates:
Platinum adheres strongly to titanium substrates, typically through electroplating. The bonding is stable and enhances the electrode’s durability under high current densities.
2. Iridium Oxide (IrO₂)
- Color:
Iridium oxide has a dark blue to black appearance, often used as a coating material. - Chemical Characteristics:
IrO₂ is highly resistant to corrosion in both acidic and alkaline environments. Its chemical inertness makes it a preferred choice for long-term applications in harsh electrolytes. - Physical Characteristics:
IrO₂ has excellent hardness and conductivity. Its melting point is approximately 2450°C, ensuring stability even under extreme conditions. - Water Electrolysis Efficiency:
As a catalyst, IrO₂ provides outstanding OER activity with low overpotential, especially in acidic conditions such as proton exchange membrane electrolysis cells (PEMECs). - Bonding with Titanium Substrates:
IrO₂ coatings are typically applied via thermal decomposition or sputtering methods, forming a strong, durable bond with titanium. This ensures high mechanical stability and long operational life.

3. Ruthenium Oxide (RuO₂)
- Color:
Ruthenium oxide appears gray to black, commonly used in mixed metal oxide (MMO) coatings. - Chemical Characteristics:
RuO₂ has excellent catalytic properties and moderate stability in acidic conditions. It performs well in alkaline media but is less stable than IrO₂ in highly acidic environments. - Physical Characteristics:
It offers good conductivity and reasonable mechanical hardness. RuO₂ has a melting point of 2334°C, ensuring adequate thermal stability for electrolysis. - Water Electrolysis Efficiency:
RuO₂ is highly active for OER, especially when used in combination with other oxides in MMO coatings. However, it is less durable in acidic conditions compared to IrO₂. - Bonding with Titanium Substrates:
When used in MMO coatings, RuO₂ forms a strong bond with titanium substrates. These coatings are optimized for high catalytic performance and durability in alkaline electrolysis.
4. Nickel (Ni)
- Color:
Nickel has a silvery metallic appearance, commonly used in cost-effective alkaline electrolysis. - Chemical Characteristics:
Nickel is moderately resistant to corrosion in alkaline conditions but is unsuitable for acidic environments due to rapid degradation. - Physical Characteristics:
Nickel is lightweight, conductive, and has a relatively low density (8.91 g/cm³) compared to noble metals. Its melting point is 1455°C. - Water Electrolysis Efficiency:
While not as efficient as platinum or oxides like IrO₂, nickel performs adequately in alkaline electrolytes. It is often used in budget-friendly setups for water splitting. - Bonding with Titanium Substrates:
Nickel has a weaker bonding efficiency with titanium compared to noble metals and oxides. However, composite techniques like nickel alloys can improve adhesion.
5. Mixed Metal Oxides (MMOs)
- Color:
MMOs exhibit varied colors, depending on their composition, typically gray or black. - Chemical Characteristics:
MMOs are engineered combinations of oxides like IrO₂, RuO₂, and others, offering tailored chemical stability for specific electrolytic environments. - Physical Characteristics:
The physical properties of MMOs vary based on their composition but are optimized for high conductivity, mechanical durability, and resistance to wear. - Water Electrolysis Efficiency:
MMOs are highly efficient for OER due to their combination of active materials. They are commonly used in both acidic and alkaline electrolyzers, balancing performance and cost. - Bonding with Titanium Substrates:
MMOs are specifically designed for strong adhesion to titanium substrates. These coatings ensure excellent mechanical and electrochemical performance over extended periods.

| Material | Color | Chemical Stability | Physical Characteristics | Electrolysis Efficiency | Bonding with Titanium |
| Platinum (Pt) | Silver-White | High in all environments | High density, excellent conductivity | High (low overpotential) | Strong (via electroplating) |
| Iridium Oxide (IrO₂) | Dark Blue/Black | High in acidic and alkaline | High hardness, excellent conductivity | High (optimized for OER) | Strong (thermal decomposition) |
| Ruthenium Oxide (RuO₂) | Gray-Black | Moderate in acidic, high in alkaline | Good conductivity, moderate hardness | Moderate (used in MMO coatings) | Strong (via MMO coatings) |
| Nickel (Ni) | Silvery | Moderate in alkaline, poor in acidic | Lightweight, good conductivity | Moderate (alkaline applications) | Weak to moderate |
| Mixed Metal Oxides | Varied (Gray/Black) | High (tailored properties) | Optimized for conductivity and durability | High (customized combinations) | Very strong |
Price Trends and Market Analysis of Key Anode Materials (2020-2024)
Analyzing the price trends of key anode materials over the past five years provides valuable insights into their economic viability for water electrolysis applications. Below is a detailed overview of the price movements for Platinum (Pt), Iridium (Ir), Ruthenium (Ru), Nickel (Ni), and Mixed Metal Oxides (MMOs) from 2020 to 2024.
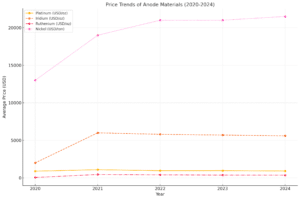
1. Platinum (Pt)
- Price Trends (2020-2024):
- 2020: The average closing price was $893.39 per troy ounce, with a peak at $1,078.44.
- 2021: Prices increased, averaging $1,088.51, reaching a high of $1,293.10.
- 2022: A slight decline occurred, with an average of $958.06 and a high of $1,153.20.
- 2023: Further decrease to an average of $965.28, peaking at $1,124.00.
- 2024: As of December 13, the price is $917.31 per troy ounce, with an average of $954.95.
Data Source: MacroTrends
- Market Analysis:
- Supply Factors: Fluctuations in mining output, particularly from South Africa, have influenced supply levels.
- Demand Factors: The automotive industry’s demand for catalytic converters and investment interests have impacted prices.
2. Iridium (Ir)
- Price Trends (2020-2024):
- 2020: Prices surpassed $2,000 per ounce, marking a significant increase.
- 2021: An all-time high was reached, exceeding $6,000 per ounce.
- 2022-2024: Prices have remained elevated, with some fluctuations, reflecting ongoing demand and limited supply.
Data Source: Daily Metal Prices
- Market Analysis:
- Supply Constraints: Limited mining sources and geopolitical factors have restricted supply.
- Demand Drivers: Applications in electronics, catalysis, and emerging technologies have sustained high demand.
3. Ruthenium (Ru)
- Price Trends (2020-2024):
- 2020: Prices were approximately $14,950 per kilogram.
- 2021-2024: Prices have shown variability, influenced by industrial demand and supply dynamics.
Data Source: Wikipedia
- Market Analysis:
- Supply Considerations: As a by-product of platinum and nickel mining, its availability is linked to the production of these metals.
- Demand Applications: Utilization in electronics, chemical industries, and potential in renewable energy technologies have influenced demand.
4. Nickel (Ni)
- Price Trends (2020-2024):
- 2020: Prices averaged around $13,000 per metric ton.
- 2021: An increase was observed, with prices reaching approximately $19,000 per metric ton.
- 2022: Prices peaked at over $21,000 per metric ton, driven by supply concerns.
- 2023-2024: Fluctuations continued, with prices influenced by geopolitical tensions and demand from the battery sector.
Data Source: Financial Times
- Market Analysis:
- Supply Dynamics: Political instability in key producing regions and increased output from countries like Indonesia have affected supply.
- Demand Factors: Nickel’s critical role in electric vehicle batteries and stainless steel production has driven demand.
5. Mixed Metal Oxides (MMOs)
- Market Growth (2023-2030):
- The global MMO electrode market was valued at $165.2 million in 2023 and is projected to reach $272.3 million by 2030, exhibiting a CAGR of 7.3%.
Data Source: Semiconductor Insight
- Market Analysis:
- Supply Factors: Dependent on the availability of constituent metals like iridium and ruthenium.
- Demand Drivers: Applications in water treatment, electroplating, and cathodic protection have spurred market growth.
The past five years have witnessed significant price volatility among these critical materials, influenced by factors such as geopolitical tensions, technological advancements, and shifts in industrial demand. Understanding these trends is essential for stakeholders in the water electrolysis industry to make informed decisions regarding material selection and cost management.
Balancing Environmental Impact and Economic Efficiency
The shift toward sustainable energy has placed water electrolysis at the forefront of green hydrogen production. However, the choice of anode materials significantly influences both the environmental impact and economic efficiency of this process. This article examines the key materials—Platinum (Pt), Iridium Oxide (IrO₂), Ruthenium Oxide (RuO₂), Nickel (Ni), and Mixed Metal Oxides (MMOs)—through the lenses of sustainability and cost-effectiveness.

1. Platinum (Pt): A High-Performance but Costly Option
Economic Efficiency:
Platinum is widely recognized for its exceptional catalytic performance, offering low overpotential and high efficiency in oxygen evolution reactions (OER). However, its high price (averaging $954.95 per ounce in 2024) and limited availability restrict its widespread adoption. The mining and refining of platinum are capital-intensive processes, contributing to its elevated costs.
Environmental Impact:
Platinum mining has a significant ecological footprint. It involves deep-earth mining and the use of toxic chemicals like cyanide, which pose risks to surrounding ecosystems. Additionally, energy-intensive extraction processes lead to high carbon emissions. While platinum’s durability reduces the need for frequent replacement, its extraction impacts offset this environmental benefit.
Conclusion:
Platinum’s performance is unmatched, but its environmental and economic costs make it suitable only for specialized applications, such as proton exchange membrane (PEM) electrolyzers.
2. Iridium Oxide (IrO₂): The Green Hydrogen Catalyst
Economic Efficiency:
Iridium Oxide is a leading material for OER in acidic environments, particularly in PEM electrolyzers. Its cost, exceeding $5,500 per ounce, stems from its rarity and complex extraction. Despite its high price, IrO₂ delivers a long operational life, reducing long-term costs in high-efficiency systems.
Environmental Impact:
Iridium’s environmental challenges mirror those of platinum. Its mining is energy-intensive and produces significant waste. However, its use in green hydrogen production aligns with global decarbonization goals, offering an indirect environmental benefit by enabling the replacement of fossil fuels.
Conclusion:
Iridium balances high initial costs with durability and efficiency, making it a critical material for transitioning to renewable energy, albeit with significant environmental trade-offs.
3. Ruthenium Oxide (RuO₂): A Cost-Effective Catalyst for Mixed Metal Oxides
Economic Efficiency:
Ruthenium Oxide is less expensive than platinum and iridium, with prices stabilizing around $360 per ounce in 2024. It is commonly used in combination with other materials in MMOs, optimizing both cost and performance. Its efficiency in alkaline environments makes it a versatile choice for cost-conscious applications.
Environmental Impact:
Like other precious metals, ruthenium’s extraction contributes to habitat destruction and waste generation. However, its integration into MMOs allows for reduced material usage, minimizing its overall environmental footprint.
Conclusion:
Ruthenium provides a practical balance between cost and environmental considerations, especially when incorporated into MMOs for alkaline water electrolysis.
4. Nickel (Ni): The Budget-Friendly Choice
Economic Efficiency:
Nickel is the most economical option among the materials discussed, with prices averaging $21,500 per metric ton in 2024. Its widespread availability and lower extraction costs make it ideal for alkaline electrolysis. However, its efficiency and stability lag behind noble metals, particularly in acidic environments.
Environmental Impact:
Nickel mining is associated with significant environmental degradation, including deforestation, soil erosion, and heavy metal contamination of water sources. Additionally, smelting and refining processes contribute to high greenhouse gas emissions. Despite these drawbacks, nickel’s abundance and recyclability offer pathways for mitigating its environmental impact.
Conclusion:
Nickel is a cost-effective choice for non-critical applications, but its environmental impact necessitates careful management and recycling initiatives.
5. Mixed Metal Oxides (MMOs): The Sustainable Hybrid
Economic Efficiency:
MMOs combine materials like IrO₂ and RuO₂ to create coatings tailored for specific applications. This approach optimizes catalytic performance while reducing reliance on expensive noble metals. MMOs are widely used in both acidic and alkaline electrolysis systems, offering an excellent balance of cost and performance.
Environmental Impact:
By reducing the quantity of rare metals required, MMOs mitigate the environmental footprint associated with mining and refining. Additionally, their durability minimizes waste and the need for frequent replacement. However, the production of MMOs involves complex manufacturing processes that require energy and chemicals.
Conclusion:
MMOs represent a promising compromise between economic efficiency and environmental sustainability, particularly for large-scale applications.
Environmental and Economic Considerations: A Comparative Summary
Economic Factors
- Platinum and Iridium Oxide: High initial costs limit their use to high-efficiency systems.
- Ruthenium Oxide and Nickel: Cost-effective options for alkaline conditions, with varying degrees of efficiency.
- MMOs: An adaptable solution, leveraging the strengths of multiple materials for cost-effective performance.
Environmental Factors
- Mining and Extraction: All materials pose environmental risks, with platinum and iridium being the most impactful.
- Sustainability: MMOs and nickel offer pathways for reducing environmental harm through resource efficiency and recycling.
- Long-Term Impact: Materials enabling green hydrogen production contribute to global decarbonization, offsetting their environmental costs.
Future Directions: Balancing Sustainability and Cost
- Material Recycling: Establishing robust recycling systems for platinum, iridium, and ruthenium can reduce dependence on mining and lower costs.
- Innovation in MMOs: Advances in MMO technology could further minimize the use of noble metals, improving both economic and environmental outcomes.
- Renewable Mining Practices: Investing in cleaner mining technologies can mitigate the environmental impact of extracting these critical materials.
- Scaling Green Hydrogen: As green hydrogen production scales, economies of scale may drive down the cost of high-performance anode materials.
The choice of anode material for water electrolysis must balance efficiency, cost, and environmental sustainability. While noble metals like platinum and iridium deliver unmatched performance, their high costs and ecological impacts restrict their use to niche applications. Nickel and ruthenium offer cost-effective alternatives, particularly for alkaline systems, while MMOs present a hybrid solution that combines performance with sustainability.
As the global energy transition accelerates, the development of more sustainable and economically viable materials will play a critical role in the widespread adoption of water electrolysis for green hydrogen production. By prioritizing recycling, technological innovation, and renewable mining practices, the industry can align economic goals with environmental responsibility, ensuring a cleaner and more sustainable future.
Conclusion
Platinum (Pt): The High-Performance Benchmark
Platinum is renowned for its exceptional catalytic activity, low overpotential, and superior performance in oxygen evolution reactions (OER). This makes it an ideal material for proton exchange membrane (PEM) electrolyzers. However, its high price (averaging $954.95 per ounce in 2024) and limited availability restrict its use to specialized, high-efficiency applications.
From an environmental perspective, platinum mining is energy-intensive and involves the use of toxic chemicals like cyanide, leading to significant habitat destruction and carbon emissions. While platinum’s durability and long operational life reduce replacement frequency, the ecological footprint of its extraction is substantial.
Iridium Oxide (IrO₂): The Catalyst for Green Hydrogen
Iridium Oxide is a leading choice for acidic environments, particularly in PEM systems. Its cost, exceeding $5,500 per ounce, reflects its rarity and the complexity of its extraction. Despite its high price, IrO₂ offers excellent durability and long-term performance, making it a cornerstone of high-efficiency electrolysis systems.
The environmental impact of iridium mirrors that of platinum, with deep-earth mining and high-energy extraction processes causing significant ecological harm. However, its role in enabling green hydrogen production helps offset these drawbacks by contributing to the decarbonization of energy systems.
Ruthenium Oxide (RuO₂): A Cost-Effective Alternative
Ruthenium Oxide strikes a balance between cost and performance. With prices around $360 per ounce in 2024, it is significantly cheaper than platinum or iridium. Often used in Mixed Metal Oxides (MMOs), ruthenium enhances the catalytic performance of alkaline systems.
Environmentally, ruthenium’s impact is tied to its status as a by-product of platinum and nickel mining. While its extraction involves fewer resources compared to primary metals, it still contributes to habitat destruction and waste generation. Incorporating ruthenium into MMOs helps mitigate these impacts by reducing the overall material requirement.
Nickel (Ni): The Economical Workhorse
Nickel is the most cost-effective option among these materials, with prices averaging $21,500 per metric ton in 2024. It is particularly effective in alkaline water electrolysis, where it provides reasonable performance at a fraction of the cost of noble metals. However, its catalytic efficiency and stability are inferior to platinum and iridium, limiting its application in acidic systems.
Nickel mining, despite its economic advantages, has significant environmental consequences. These include deforestation, soil erosion, and heavy metal contamination of nearby water sources. Smelting and refining processes also produce considerable greenhouse gas emissions. Nevertheless, nickel’s abundance and recyclability offer opportunities to mitigate its environmental impact.
Mixed Metal Oxides (MMOs): The Hybrid Solution
MMOs combine materials like IrO₂ and RuO₂ to optimize performance while reducing reliance on expensive noble metals. This adaptability makes them an attractive choice for both acidic and alkaline systems. By balancing cost, efficiency, and durability, MMOs are becoming a preferred solution for large-scale applications.
From an environmental standpoint, MMOs reduce the demand for rare metals, mitigating the ecological footprint associated with their extraction. Their durability further minimizes waste and the need for frequent replacement, aligning with sustainability goals.
The selection of anode materials for water electrolysis must balance performance, cost, and sustainability. While noble metals like platinum and iridium are indispensable for high-efficiency systems, their environmental and economic costs limit their application. Nickel and ruthenium offer affordable alternatives, particularly for alkaline systems, and MMOs present an innovative hybrid solution.
As green hydrogen production scales globally, the development of more sustainable and economically viable materials will be critical. By prioritizing recycling, technological innovation, and cleaner mining practices, the water electrolysis industry can align its goals with environmental responsibility, driving the transition to a cleaner and more sustainable energy future.