Platinum coating refers to the process of applying a thin layer of platinum onto a surface—typically a metal like titanium—to enhance its physical and chemical properties. Platinum is a noble metal known for its resistance to corrosion, high melting point, and excellent electrical conductivity. These characteristics make it an ideal material for use in harsh environments, where other metals might degrade over time.
Introduction
Platinum coating is widely recognized for its exceptional properties, including high corrosion resistance, excellent conductivity, and durability. When applied to titanium anodes, platinum significantly enhances the performance of various industrial processes. Titanium anodes are used in critical applications such as electrolysis, water treatment, and electroplating, where the material’s durability and efficiency are paramount. By adding a thin layer of platinum, the anode becomes much more resistant to corrosion, has a longer lifespan, and offers improved electrochemical performance. This article will explore the concept of platinum coating, its applications, benefits, and why it’s a crucial component for titanium anodes in various industries.
What is Platinum Coating?
Platinum coating refers to the process of applying a thin layer of platinum onto a surface—typically a metal like titanium—to enhance its physical and chemical properties. Platinum is a noble metal known for its resistance to corrosion, high melting point, and excellent electrical conductivity. These characteristics make it an ideal material for use in harsh environments, where other metals might degrade over time.
The application of platinum as a coating material is primarily done through methods such as electroplating, sputtering, or chemical vapor deposition (CVD). The choice of method depends on the desired thickness of the coating, the substrate material (like titanium), and the intended application.
In comparison to other coating materials, such as iridium or gold, platinum has the advantage of maintaining its structural integrity even under extreme conditions, such as high temperatures and aggressive chemical environments. This makes platinum-coated surfaces not only more durable but also more effective in maintaining their functionality for long periods.
Introduction to Platinum’s Properties and Its Role in Titanium Anodes
Platinum, a precious metal, is highly valued for its unique combination of properties that make it ideal for demanding industrial applications. Here’s a closer look at why platinum is so exceptional:

Key Properties of Platinum:
- Outstanding Corrosion Resistance:
Platinum is one of the most corrosion-resistant materials, capable of withstanding harsh chemicals and extreme environmental conditions. This property makes platinum a preferred choice for electrochemical applications, where other metals might degrade over time. - High Melting Point:
Platinum has a high melting point (1,768°C), which allows it to retain its integrity even at elevated temperatures. This is crucial for industries like electroplating, fuel cells, and water treatment, where high-temperature conditions are common. - Excellent Electrical Conductivity:
Platinum is an excellent conductor of electricity, which is essential for its role as an electrode material. Its high conductivity ensures efficient electron transfer during electrochemical reactions, contributing to faster and more efficient processes. - Catalytic Properties:
Platinum is also known for its catalytic properties, which enhance reactions without being consumed in the process. This characteristic makes platinum a key component in catalytic applications like fuel cells and chemical reactors.
How Platinum Coating Enhances Titanium Anodes
Platinum-coated titanium anodes leverage these unique properties of platinum to offer superior performance in electrochemical processes. Here’s a breakdown of how platinum coatings significantly improve titanium anodes:
1. Enhanced Corrosion Resistance
- Before Coating: Titanium, while inherently corrosion-resistant, may still suffer degradation when exposed to highly aggressive environments, such as chlorine or acidic conditions in electrochemical applications.
- After Platinum Coating: The platinum coating provides a secondary layer of protection, significantly increasing the anode’s lifespan and preventing any electrochemical corrosion. This makes platinum-coated titanium anodes ideal for long-term use in harsh chemical environments.
2. Improved Electrical Conductivity
- Before Coating: Titanium, although a decent conductor, may not offer the same level of conductivity required for high-efficiency electrochemical reactions. This could result in higher energy consumption and slower processes.
- After Platinum Coating: Platinum’s superior conductivity allows for smoother electron transfer during electrochemical reactions, improving energy efficiency and reaction speed. This is particularly advantageous in applications such as electroplating, water treatment, and fuel cells.
3. Increased Durability and Longevity
- Before Coating: Titanium anodes can wear down over time, especially in high-current applications. The titanium base material is durable but may eventually degrade, especially in the presence of highly reactive substances.
- After Platinum Coating: The platinum coating increases the mechanical and electrochemical durability of the titanium anode, ensuring a longer lifespan even under extreme conditions. Platinum-coated titanium anodes can endure high currents and extended usage without significant degradation, reducing replacement costs.
4. Higher Performance in Electrochemical Reactions
- Before Coating: Without a platinum coating, titanium anodes may exhibit sluggish or incomplete electrochemical reactions, especially in processes like electrolysis and water treatment.
- After Platinum Coating: Platinum acts as a catalyst, enhancing the rate and efficiency of electrochemical reactions. In processes like electrolysis (used in chlorine production or water disinfection), platinum-coated titanium anodes help achieve higher reaction rates and better overall process efficiency.
Platinum Coating vs. Other Coating Materials
While platinum is an exceptional material for titanium anodes, it is important to consider how it compares with other coating materials such as iridium, gold, and mixed metal oxides (MMO). Let’s take a closer look at these materials:
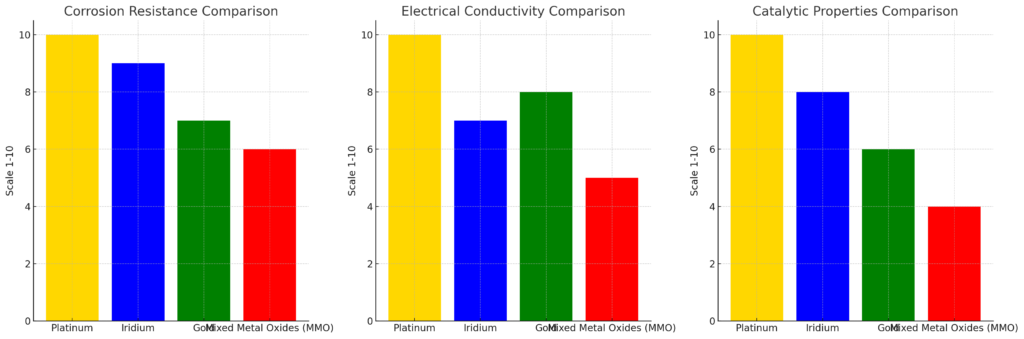
1. Platinum vs. Iridium
- Platinum: Known for its high corrosion resistance, platinum outperforms most metals in extreme conditions. It is more expensive, but its superior electrical conductivity and catalytic properties often make it the better choice for critical applications.
- Iridium: Iridium is another noble metal with excellent corrosion resistance, especially in chlorine environments. However, iridium is less conductive than platinum and does not exhibit the same catalytic efficiency, which can result in lower performance for certain applications. While iridium coatings can be more cost-effective, platinum often remains the superior choice for high-efficiency processes.
2. Platinum vs. Gold
- Platinum: While gold has a high resistance to corrosion and is highly conductive, it is softer than platinum, making it less durable in high-wear applications. Additionally, gold is often more expensive than platinum in some markets, despite its lower durability and performance in electrochemical environments.
- Gold: Gold coatings may be useful in certain low-load or decorative applications, but they are not ideal for heavy-duty electrochemical processes where high durability and conductivity are required. Platinum coatings are better suited for these demanding conditions.
3. Platinum vs. Mixed Metal Oxides (MMO)
- Platinum: Platinum coatings are highly effective in a wide range of electrochemical processes due to their high conductivity and catalytic properties. However, platinum is a more expensive material.
- MMO: MMO coatings are a more cost-effective option for titanium anodes and provide good performance in some electrochemical applications, especially in low-temperature, low-current environments. However, MMOs are generally less efficient than platinum at high currents or extreme conditions. MMOs also have lower stability and can degrade over time, especially in chlorine production applications.
Advantages of Platinum Coatings:
- Superior durability and longevity, making it ideal for critical and high-load applications.
- Higher efficiency in electrochemical processes, reducing energy consumption and improving process speeds.
- Excellent resistance to a broad range of chemicals, including chlorine, making platinum-coated titanium anodes perfect for industries like water treatment and electrolysis.
Disadvantages of Platinum Coatings:
- Higher cost, which may be a concern for certain large-scale or less-critical applications. However, the increased lifespan and improved performance often offset the higher initial cost in demanding industries.
| Property | Platinum Coated Titanium Anode | Iridium Coated Titanium Anode | Gold Coated Titanium Anode | Mixed Metal Oxides (MMO) Coated Titanium Anode |
| Corrosion Resistance | Extremely high, ideal for harsh chemical environments | Extremely high, especially in chlorine environments | High, but softer and less durable under certain conditions | Good, suitable for low to medium load environments |
| Electrical Conductivity | Very high, enabling efficient electron transfer | Lower, conductivity is less than platinum | High, but less stable in electrochemical environments | Low, not suitable for high-current environments |
| Catalytic Properties | Excellent, enhances reaction speed and efficiency | Good, but less effective than platinum | Moderate, mainly used for decorative and low-load applications | Weak, suitable for simpler reactions |
| Durability and Longevity | Very high, ideal for long-term use | High, but not as durable as platinum | Low, softer metal prone to wear under high load and current | Medium, suitable for low-temperature, low-current applications |
| Cost | High, due to precious metal costs | Relatively high, but cheaper than platinum | High, as gold is a precious metal and unsuitable for high-load environments | Low, but performance is relatively poor |
| Ideal Applications | High-load, high-efficiency electrochemical processes (e.g., electrolysis, water treatment) | Chlorine environments, but lower efficiency | Low-load, decorative applications | Low-temperature, low-current environments |
| Overall Advantages | Superior performance, suitable for demanding electrochemical reactions | Lower cost but lower catalytic efficiency and durability | Mainly for low-load applications, less durable | Lower cost, suitable for light-duty applications, but not ideal for high-intensity use |
Platinum coatings significantly enhance the performance of titanium anodes, particularly in harsh electrochemical environments where longevity, efficiency, and resistance to corrosion are paramount. While the higher cost of platinum may be a consideration, its exceptional properties make it an investment that can lead to greater process efficiency and reduced maintenance costs in the long term.
When compared to other coatings like iridium, gold, and MMO, platinum stands out for its superior conductivity, durability, and catalytic properties, especially in high-load, high-performance applications. For industries such as electroplating, water treatment, and fuel cell technology, platinum-coated titanium anodes offer unmatched benefits, making them an indispensable choice for achieving optimal performance in electrochemical processes.
How is Platinum Coated on Titanium?
The process of applying platinum to titanium is typically done using one of three main techniques: electroplating, sputtering, or chemical vapor deposition (CVD).
- Electroplating:
This is the most common method used to coat titanium with platinum, especially for anodes. In electroplating, titanium is immersed in a solution containing platinum salts. An electrical current is passed through the solution, which causes platinum ions to deposit onto the surface of the titanium. The thickness of the platinum coating can be controlled by adjusting the plating time and current density. Electroplating is a cost-effective method for applying thin platinum layers (usually between 2-5 microns) and is widely used for manufacturing platinum-coated titanium anodes.
- Sputtering:
In sputtering, platinum atoms are ejected from a platinum target material and deposited onto the titanium substrate in a vacuum chamber. This technique is particularly useful for creating uniform coatings at the nanoscale level. While sputtering allows for precise control over the thickness and quality of the coating, it is typically more expensive and less commonly used for bulk production of titanium anodes.
- Chemical Vapor Deposition (CVD):
CVD involves introducing a platinum precursor gas into a reaction chamber, where it reacts and deposits a thin platinum layer onto the titanium surface. This method provides excellent adhesion and uniformity of the coating, especially for more complex geometries. However, CVD is more expensive and typically used for specialized applications where high-quality, thick coatings are required.
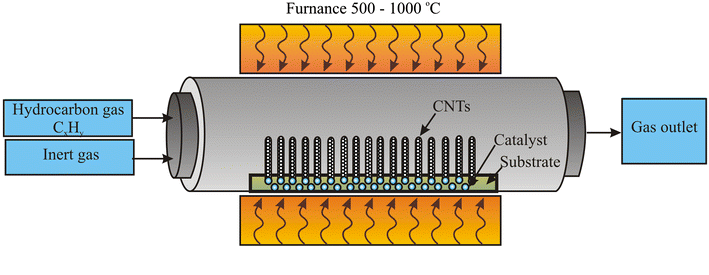
Each of these methods has its own advantages, but electroplating remains the most economical and widely used approach, particularly in industrial settings.
Platinum Coating for Titanium Anodes: Applications and Benefits
Platinum-coated titanium anodes are widely used in various industries due to the enhanced properties that platinum imparts to the titanium substrate. Some of the key benefits include:

Advantages of Platinum Coating on Titanium Anodes
There are several reasons why platinum-coated titanium anodes are preferred in various industrial applications:
- High Corrosion Resistance:
Platinum’s excellent resistance to corrosion in aggressive environments such as chlorine gas or acids makes it an ideal material for coatings in electrolytic processes. This durability ensures that the titanium anode remains functional over an extended period, even in harsh chemical settings. - Long Service Life:
The platinum layer significantly extends the lifespan of titanium anodes. The combination of platinum’s resistance to wear and the titanium’s strength ensures that the anode remains effective for years, even in demanding applications. - Superior Conductivity:
Platinum’s high electrical conductivity improves the performance of the anode in electrochemical processes, reducing energy losses and improving the efficiency of reactions. - Cost-Effectiveness:
While platinum is expensive, its use as a coating material rather than the base material allows industries to leverage the metal’s benefits without facing prohibitive costs.
Applications of Platinum-Coated Titanium Anodes in Various Industries
Platinum-coated titanium anodes are used in a variety of industrial applications, including:
- Water Treatment:
In electrolysis processes for water treatment, platinum-coated titanium anodes are used to generate chlorine and oxygen efficiently. The anodes’ high corrosion resistance ensures that they remain effective in long-term, high-temperature, and high-chlorine environments. - Electroplating:
Platinum-coated titanium anodes are commonly used in electroplating to provide a stable and durable electrode surface. Their high conductivity and corrosion resistance ensure that they perform well even in high-current electroplating processes. - Fuel Cells:
In hydrogen fuel cells, platinum-coated titanium anodes are essential for promoting the efficient electrochemical reactions required for energy production. Platinum’s excellent catalytic properties enhance the performance of these fuel cells. - Chlor-Alkali Industry:
In the production of chlorine and caustic soda, platinum-coated titanium anodes are used in electrolytic cells. The platinum coating ensures that the anodes maintain their integrity even in the aggressive chlorine environment.
Detailed Analysis of the Environmental Benefits of Platinum Coating
Platinum-coated titanium anodes offer significant environmental benefits due to their superior properties in electrochemical applications. Let’s break down the key environmental advantages of platinum coatings:

1. Enhanced Durability and Longevity
- Environmental Impact: Platinum’s exceptional durability means that platinum-coated titanium anodes last longer than other alternatives. This reduces the need for frequent replacements, which leads to fewer resources being consumed in the manufacturing process. Additionally, the reduction in waste from worn-out anodes helps in minimizing environmental pollution.
- Longer Product Life Cycle: A longer lifespan for platinum-coated anodes means fewer materials need to be mined, manufactured, and disposed of. This results in a lower carbon footprint over time compared to frequently replaced anodes made from less durable materials.
2. Energy Efficiency in Electrochemical Processes
- Environmental Impact: Platinum’s high electrical conductivity ensures that electrochemical reactions are more efficient. In applications such as water treatment or electroplating, more efficient processes mean less energy consumption, contributing to a reduction in greenhouse gas emissions and the overall environmental footprint.
- Reduced Carbon Emissions: When platinum-coated titanium anodes are used in electrolysis processes (e.g., chlorine production, water splitting for hydrogen generation), the energy efficiency leads to lower carbon emissions, helping meet sustainability goals in industries like green energy production.
3. Resistance to Corrosion in Harsh Chemical Environments
- Environmental Impact: Platinum’s outstanding corrosion resistance allows it to withstand aggressive environments like chlorine production, which would otherwise degrade less stable materials. This minimizes the risk of hazardous material leakage into the environment, ensuring that the surrounding ecosystems are not exposed to toxic chemicals.
- Safe Handling and Reduced Hazardous Waste: In industries like water treatment, platinum-coated anodes reduce the need for toxic chemicals that could harm the environment. By improving the stability of the electrochemical process, platinum coatings help mitigate harmful byproducts.
Detailed Analysis of the Cost Factors of Platinum Coating
While platinum is an excellent material for enhancing the performance of titanium anodes, its cost is often a significant consideration. Let’s delve into the factors that influence the price of platinum coatings:

1. Precious Metal Costs
- Market Volatility: Platinum is a precious metal, and its price is subject to fluctuations based on supply and demand dynamics in the global market. Prices for platinum can be volatile, which directly affects the cost of platinum-coated titanium anodes. This volatility often means that the cost can be unpredictable, making it challenging to budget for large-scale installations.
- Extraction and Refining Costs: The extraction and refining of platinum require complex and energy-intensive processes. Mining platinum involves environmentally impactful procedures, including the use of chemicals and large amounts of energy, which increases the overall cost of the material. These costs are ultimately passed on to the end consumer.
2. Coating Application Costs
- Labor and Technology: Applying platinum coatings to titanium anodes is a delicate process that involves advanced technology, such as electroplating or sputtering, to ensure uniformity and high-quality adhesion. The labor costs associated with these specialized techniques contribute to the overall price of the coated anodes.
- Specialized Equipment: High-end equipment and machinery are required for the platinum coating process. These systems are expensive to purchase and maintain, which also adds to the cost of platinum-coated anodes.
3. Performance-Based Value
- Long-Term Investment: While platinum coatings are more expensive upfront, their superior performance and longevity often make them a cost-effective option over time. The reduced frequency of replacements, combined with the energy efficiency they provide, can justify the higher initial investment. In applications where downtime is costly, the durability of platinum-coated titanium anodes ensures that companies don’t have to frequently replace components, leading to lower maintenance costs in the long run.
- Total Cost of Ownership: From an operational perspective, the total cost of ownership (TCO) of platinum-coated anodes may be lower than alternatives due to reduced energy consumption and longer lifespan. For industries that require high uptime and reliability, the benefits of platinum coatings can outweigh the initial price difference.
Comprehensive Analysis of Platinum Coating’s Environmental Benefits and Cost Factors
When evaluating platinum-coated titanium anodes, it’s crucial to balance both the environmental benefits and the cost considerations.
Environmental Benefits vs. Costs
- Environmental Benefit: The environmental advantages of platinum coatings are clear: they enhance the durability and efficiency of electrochemical processes, reduce energy consumption, and minimize waste. These factors make platinum-coated anodes an environmentally responsible choice, especially in energy-intensive industries like water treatment and electroplating.
- Cost Consideration: However, the high cost of platinum is a limiting factor. The precious metal’s price, volatility in the market, and the expensive coating process all contribute to a higher upfront cost for platinum-coated anodes. This may deter some businesses from choosing platinum over more affordable materials.
Sustainability vs. Budget Constraints
- Sustainability: Platinum coatings contribute to sustainability efforts by improving process efficiency, reducing energy consumption, and minimizing environmental impact. Industries aiming to meet sustainability targets can justify the investment in platinum-coated anodes for the long-term environmental benefits.
- Budget Constraints: For companies with budget constraints or those working in less demanding environments, the initial cost of platinum may be prohibitive. In these cases, alternatives like iridium coatings or mixed metal oxides (MMO) coatings, though less efficient in some respects, can provide a more cost-effective solution without the higher upfront investment of platinum.
Comparison with Other Coating Materials
- Iridium Coatings: Iridium, like platinum, is a noble metal with excellent corrosion resistance, particularly in chlorine environments. It is often used as a more cost-effective alternative to platinum. However, iridium coatings do not offer the same electrical conductivity or catalytic properties, meaning the overall performance is slightly lower. For certain industrial applications, iridium may provide an adequate balance between cost and performance.
- Mixed Metal Oxides (MMO): MMO coatings are the most cost-effective alternative to platinum. They offer good performance in mild conditions and are widely used in lower-cost applications. However, MMOs are less durable and efficient compared to platinum, especially in high-load, high-current electrochemical processes. While cheaper, they do not provide the same longevity or energy savings that platinum coatings offer.
- Gold Coatings: Gold offers excellent corrosion resistance but is much softer than platinum, leading to shorter lifespans in high-stress applications. Gold is generally more expensive than other alternatives like iridium but is not suitable for high-performance or high-load applications where platinum excels.
Conclusion
In conclusion, platinum-coated titanium anodes offer a range of advantages, including enhanced corrosion resistance, increased durability, and improved electrical conductivity. These properties make them essential in applications such as water treatment, electroplating, and fuel cell technology. Although platinum is a precious metal, using it as a coating on titanium provides a cost-effective solution while maintaining high performance. If you’re looking for high-quality titanium anodes for your industrial applications, consider the benefits of platinum coatings for superior long-term performance and efficiency.