Platinum electrodes are a critical investment for industries requiring precision and resilience in challenging environments. From electroplating to electrolytic hydrogen production, their superior performance ensures high-quality, long-lasting results.
Introduction
Platinum electrodes, renowned for their durability, chemical resistance, and unmatched versatility, are critical in industries where precision and reliability are paramount. From electroplating and water treatment to electrolytic hydrogen production, they outshine other materials in demanding conditions. But when is it worth investing in a platinum electrode, and how does it compare to alternatives like titanium electrodes?

This guide provides insights for procurement specialists, researchers, and industry professionals into the ideal uses, benefits, and comparative advantages of platinum electrodes.
The Properties of Platinum: A Comprehensive Analysis
Platinum is a rare and precious metal that is renowned for its remarkable physical and chemical properties. Its unique characteristics make it indispensable in a variety of industrial, technological, and scientific applications. Below is a detailed overview of platinum’s physical and chemical properties.
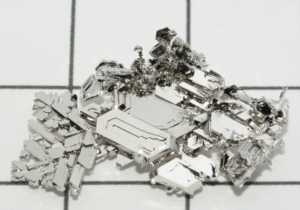
Physical Properties of Platinum
1. Density
Platinum is an extremely dense metal with a density of 21.45 g/cm³, making it one of the heaviest elements. This high density contributes to its durability and resistance to wear, essential for high-stress applications like automotive catalytic converters and jewelry.
2. Melting and Boiling Points
Platinum has a high melting point of 1,768°C (3,214°F) and a boiling point of 3,827°C (6,920°F). This thermal stability allows it to maintain structural integrity in high-temperature environments, such as in laboratory equipment and industrial furnaces.
3. Electrical Conductivity
Platinum is an excellent conductor of electricity, with a conductivity of 9.43 x 10⁶ S/m at room temperature. This property is particularly valuable in electronic components, where consistent and reliable electrical performance is critical.
4. Thermal Conductivity
With a thermal conductivity of 71.6 W/m·K, platinum effectively transfers heat. This is why it is used in applications like thermocouples and heat exchangers, where precise temperature measurements and control are required.
5. Ductility and Malleability
Platinum is highly ductile and malleable, meaning it can be drawn into thin wires or beaten into thin sheets without breaking. These attributes make it easy to fabricate into intricate designs, such as in fine jewelry or complex electrode structures.
6. Luster and Appearance
Platinum has a silvery-white metallic luster that resists tarnishing, even after long-term exposure to the environment. This aesthetic quality makes it highly sought after for luxury items like watches and jewelry.
Chemical Properties of Platinum
1. Chemical Inertness
Platinum is one of the most chemically inert metals, showing exceptional resistance to oxidation, corrosion, and tarnishing. It does not react with oxygen at any temperature and remains stable in the presence of acids, bases, and most other chemicals.
2. Resistance to Acids
One of platinum’s most striking features is its resistance to acid attack. While it resists most acids, it can dissolve in aqua regia (a mixture of concentrated nitric acid and hydrochloric acid), which produces chloro-platinic acid.
3. Catalytic Activity
Platinum is an outstanding catalyst, playing a vital role in chemical reactions such as hydrogenation, oxidation, and reforming processes. Its surface readily adsorbs and activates molecules, facilitating reactions in automotive catalytic converters, chemical production, and fuel cells.
4. Alloy Formation
Platinum readily forms alloys with other metals like gold, palladium, and rhodium. These alloys are used to enhance specific properties, such as hardness and resistance to wear, in various industrial and decorative applications.
5. Reactions with Halogens
Platinum can react with halogens like chlorine, fluorine, and bromine under certain conditions, forming halide compounds. These reactions typically require elevated temperatures or specific catalysts.
6. Electrochemical Stability
Platinum exhibits remarkable stability in electrochemical environments, which is why it is a preferred material for electrodes in electrolysis, sensors, and batteries. It resists corrosion and maintains consistent performance over time.
Unique Attributes of Platinum
1. Noble Metal Characteristics
As a noble metal, platinum is resistant to oxidation and corrosion, even under extreme conditions. This characteristic contributes to its longevity in industrial and scientific applications.
2. Non-Toxicity and Biocompatibility
Platinum is non-toxic and biologically inert, making it suitable for medical applications such as pacemakers, dental implants, and chemotherapy drugs.
3. Workability
Platinum’s workability allows it to be processed into various forms, including sheets, wires, and powders. This versatility supports its use in diverse industries, from electronics to jewelry.

Comparison of Platinum with Gold, Silver, and Titanium
| Property | Platinum | Gold | Silver | Titanium |
| Corrosion Resistance | Excellent: Resists most acids and bases. | Excellent: Resists tarnish and corrosion. | Moderate: Prone to tarnishing in sulfurous or humid environments. | Good: Resistant to many forms of corrosion but weaker in chloride environments. |
| Catalytic Efficiency | Outstanding: Excels in reactions like hydrogen evolution and oxidation. | Limited: Rarely used as a catalyst. | Moderate: Some catalytic activity in specific reactions, like ethylene oxide production. | Poor: Limited catalytic capabilities; mainly used as a substrate. |
| Melting Point | High: 1,768°C (3,214°F). | Lower: 1,064°C (1,947°F). | Lower: 961°C (1,763°F). | Higher: 1,668°C (3,034°F). |
| Electrical Conductivity | Excellent: Conducts electricity efficiently (9.43 x 10⁶ S/m). | Excellent: Slightly higher than platinum. | Highest: Best electrical conductor (6.30 x 10⁷ S/m). | Moderate: Lower than platinum and precious metals. |
| Thermal Stability | Outstanding: Stable at high temperatures. | Good: Stable under normal conditions but less effective in extreme heat. | Poor: Prone to oxidation at high temperatures. | Good: Retains stability in moderate thermal environments. |
| Density | High: 21.45 g/cm³. | High: 19.32 g/cm³. | Moderate: 10.49 g/cm³. | Low: 4.51 g/cm³, making it lightweight. |
| Cost | Very High: Rare and expensive. | Very High: Also rare and expensive. | Moderate: Cheaper than gold and platinum. | Low: Affordable and widely available. |
| Mechanical Strength | High: Durable under stress and wear. | Moderate: Softer, less durable under mechanical stress. | Low: Ductile but prone to deformation. | Excellent: Strong and lightweight. |
| Oxidation Resistance | Exceptional: Does not oxidize even at high temperatures. | Good: Stable in normal atmospheric conditions. | Poor: Oxidizes readily, forming tarnish in air. | Good: Forms a protective oxide layer but can degrade in specific environments. |
Key Takeaways:
- Platinum vs. Gold: Platinum is better suited for high-temperature and catalytic applications due to its higher melting point and catalytic efficiency. Gold is primarily used in applications where corrosion resistance and aesthetic value are paramount.
- Platinum vs. Silver: While silver excels in electrical conductivity, it falls short in corrosion resistance and thermal stability, making platinum the preferred choice in harsh environments.
- Platinum vs. Titanium: Titanium’s lightweight and affordability make it an excellent substrate material, but it cannot rival platinum’s catalytic efficiency and electrical conductivity.
Analysis of Platinum Coating Adhesion on Titanium Substrate
Platinum coatings are widely used on various substrates, but titanium stands out as an exceptional base material due to its unique combination of physical and chemical properties. Below is an analysis comparing the adhesion, compatibility, and cost-effectiveness of platinum coatings on titanium versus other common substrates.

Comparison of Platinum Coating Adhesion on Different Substrates
|
|
|
|
|
|||||
| Titanium (Ti) | Excellent | – Forms a strong bond due to titanium’s oxide layer. – Corrosion-resistant. – Lightweight yet durable. |
|
|
|||||
| Stainless Steel | Good | – Readily available and affordable. – Strong mechanical properties. |
|
|
|||||
| Graphite | Moderate | – Excellent conductivity.
– Lightweight and cost-effective. |
– Brittle and prone to structural degradation. – Limited adhesion due to weak bonding surface. |
Low: Limited durability and application scope. | |||||
| Nickel (Ni) | Good | – Good conductivity and corrosion resistance. | – Higher cost compared to stainless steel.
– Adhesion weaker than titanium in aggressive environments. |
Moderate: Good adhesion but less versatile. | |||||
| Copper (Cu) | Fair | – High conductivity. – Easy to machine and affordable. |
– Highly reactive, prone to corrosion. – Weak adhesion due to chemical mismatch with platinum. |
Low: Poor durability in harsh environments. |
Why Titanium Provides the Best Adhesion for Platinum Coatings
- Oxide Layer Interaction:
- Titanium naturally forms a stable and protective oxide layer that enhances the bonding of platinum coatings through chemical and mechanical anchoring.
- Corrosion Resistance:
- Titanium is highly resistant to corrosion, particularly in acidic or chloride-rich environments, preserving the integrity of both the substrate and the platinum coating.
- Mechanical Strength:
- The combination of titanium’s lightweight nature and high strength ensures durability in applications requiring mechanical stress.
- Thermal Expansion Compatibility:
- Titanium’s thermal expansion coefficient is closer to that of platinum, minimizing stress and delamination during temperature fluctuations.
Cost-Effectiveness Analysis
| Evaluation Factor | Titanium | Stainless Steel | Graphite | Nickel | Copper |
| Adhesion Strength | Excellent | Good | Moderate | Good | Fair |
| Corrosion Resistance | Excellent | Moderate | Poor | Good | Poor |
| Mechanical Durability | High | High | Low | Moderate | Low |
| Cost | Moderate | Low | Low | Moderate | Low |
| Overall Cost-Effectiveness | High | Moderate | Low | Moderate | Low |
Platinum coatings on titanium substrates offer the best adhesion strength due to the chemical compatibility and mechanical durability of titanium. Compared to other substrates, titanium provides the optimal balance of performance and cost, making it the most cost-effective solution for applications requiring high-performance platinum anodes.
Applications of Platinum Electrodes and Their Role in Key Fields
Platinum electrodes are integral in various industrial and scientific fields due to their unmatched catalytic activity, chemical inertness, and superior conductivity. Their roles span from facilitating electrochemical reactions in energy systems to enabling precise sensor technology in medical diagnostics. Below is an in-depth exploration of the application scenarios for platinum electrodes, detailing their functions with chemical reactions and supported by scientific insights from relevant literature.
1. Electrolysis of Water: Hydrogen Production
Overview:
Hydrogen production via water electrolysis is a cornerstone of clean energy technologies. Platinum electrodes are essential in proton exchange membrane (PEM) electrolyzers due to their superior catalytic properties.
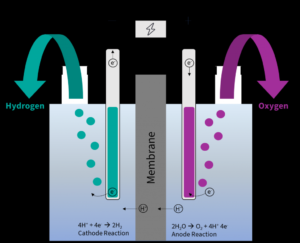
Role of Platinum Electrodes:
- Catalytic Activity: Platinum acts as a highly efficient catalyst for both the hydrogen evolution reaction (HER) at the cathode and the oxygen evolution reaction (OER) at the anode.
- Electrode Reactions:
- At the Cathode (HER):
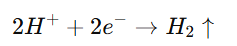 Platinum’s surface binds hydrogen intermediates with optimal strength, facilitating electron transfer and hydrogen molecule desorption.
Platinum’s surface binds hydrogen intermediates with optimal strength, facilitating electron transfer and hydrogen molecule desorption.
- At the Anode (OER):
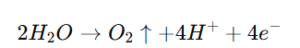 The anode reaction involves the oxidation of water, for which platinum’s stability under high anodic potentials is critical.
The anode reaction involves the oxidation of water, for which platinum’s stability under high anodic potentials is critical.
- Research Evidence:
A study published in Nature Catalysis highlights that platinum’s d-band center enables optimal adsorption of H+^+, enhancing the kinetics of HER. Additionally, platinum’s resistance to corrosion ensures its durability in acidic environments common in PEM cells.
Significance:
- High Efficiency: Platinum electrodes minimize energy losses during electrolysis, maximizing hydrogen output.
- Sustainability: By enabling clean hydrogen production, platinum contributes to decarbonization goals.
2. Electroplating: Precision Coating Applications
Overview:
Electroplating involves the deposition of a metal layer onto a substrate for improved appearance, durability, and corrosion resistance. Platinum electrodes are vital in high-precision electroplating systems, especially for precious metals like gold and rhodium.

Role of Platinum Electrodes:
- Uniform Current Distribution: Platinum electrodes maintain a stable current density, ensuring even metal deposition across complex geometries.
- Chemical Stability: Their inertness prevents contamination of the electrolyte, preserving the purity of the deposited layer.
- Electrode Reactions:
- At the Cathode (Metal Deposition):
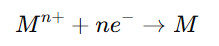
- For example, in gold plating:
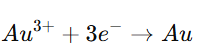
- At the Anode:
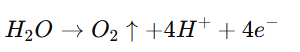
- At the Cathode (Metal Deposition):
- Research Evidence:
Research in Electrochimica Acta indicates that platinum electrodes extend the operational life of plating systems by minimizing undesirable side reactions, such as electrolyte degradation.
Significance:
- Enables precision coatings critical in electronics and luxury goods.
- Ensures product quality and consistency in industrial applications.
3. Chlorine Generation for Water Treatment
Overview:
Chlorine production through electrolysis is essential for water disinfection in municipal and industrial systems. Platinum electrodes are the preferred choice in electrolytic chlorine generators.
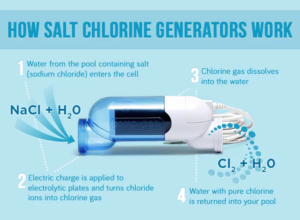
Role of Platinum Electrodes:
- Catalytic Chlorine Evolution: Platinum facilitates the chlorine evolution reaction (CER) with high efficiency and stability.
- Electrode Reactions:
- At the Anode:
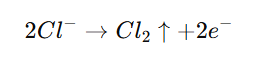
- At the Cathode:
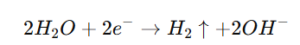
- Material Properties: Platinum’s resistance to chloride-induced corrosion ensures prolonged operational life, even in high-concentration brine solutions.
- Research Evidence:
According to the Journal of Applied Electrochemistry, platinum-coated titanium electrodes outperform mixed-metal oxide (MMO) electrodes in terms of efficiency and durability under harsh electrolytic conditions.
Significance:
- Produces chlorine efficiently for large-scale water disinfection.
- Operates reliably in corrosive environments, minimizing maintenance costs.
4. Electrochemical Sensors and Analytical Applications
Overview:
Platinum electrodes are a cornerstone in electrochemical sensors used for detecting gases, ions, and biomolecules. Their high stability and responsiveness make them ideal for medical, environmental, and industrial diagnostics.

Role of Platinum Electrodes:
- Signal Sensitivity: Platinum enhances electron transfer in redox reactions, providing accurate and sensitive readings.
- Reaction Example:
- For oxygen detection:
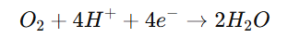
- Research Evidence:
A study in Analytical Chemistry shows that platinum electrodes improve the sensitivity and selectivity of glucose sensors by facilitating the oxidation of glucose to gluconic acid.
Significance:
- Enables the detection of trace elements with high precision.
- Integral to medical diagnostics (e.g., glucose monitoring) and environmental monitoring.
5. Catalytic Converters in Automotive Applications
Overview:
Platinum electrodes serve as catalysts in automotive catalytic converters, which reduce harmful emissions by promoting redox reactions.

Role of Platinum Electrodes:
- Catalytic Efficiency: Platinum facilitates the oxidation of carbon monoxide and hydrocarbons, as well as the reduction of nitrogen oxides.
- Reaction Examples:
- Oxidation of Carbon Monoxide:
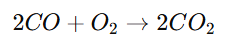
- Reduction of Nitrogen Oxides:
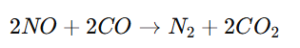
- Research Evidence:
Studies in Catalysis Science & Technology highlight platinum’s ability to adsorb reactants, weaken chemical bonds, and accelerate reaction rates without degradation.
Significance:
- Reduces environmental pollution.
- Meets stringent emission regulations globally.
Platinum electrodes are pivotal in a wide range of applications due to their unmatched stability, catalytic activity, and durability. Their contributions extend beyond facilitating key reactions to enabling cleaner energy, safer water, and more precise diagnostics. These advantages are supported by detailed reaction mechanisms and corroborated by extensive research, confirming their indispensable role in modern technology and industrial processes.
Conclusion
Platinum electrodes are a critical investment for industries requiring precision and resilience in challenging environments. From electroplating to electrolytic hydrogen production, their superior performance ensures high-quality, long-lasting results.
If you’re seeking reliable electrode technology, Ehisen Anode offers expertise and tailored solutions to optimize your operations. Visit Ehisen Anode to explore the best in platinum and titanium electrode technology.
At Ehisen Anode, we specialize in premium platinum electrode solutions:
- Rigorous Quality Control: Ensures purity, specification adherence, and durability.
- Customized Solutions: Tailored designs for specific industrial or research applications.
- Expert Consultation: Professional guidance to select the ideal electrode for your needs.







2 Responses
Your writing fosters attentive observation. Ordinary details become meaningful through phrasing and rhythm, creating moments of insight, reflection, and quiet presence.
Thank you for your recognition. As a manufacturer of titanium anodes, we will regularly share more information, hoping it will be of assistance to you.